




Regional Resource Hub: Health Technology Assessment India (HTAIn)
The Ministry of Health and Family Welfare, Department of Health Research under its research governance mandate has set up Health Technology Assessment India (HTAIn) as an attached office to systematically evaluate the available and new health technologies in India to prioritize national health spending. It is a tool that can be used in decision making for efficient use of limited health budge, ensure universal health coverage by providing people access to the quality health care thus reducing their out-of-pocket expenditures (OOPE) on health. More information available on https://htain.dhr.gov.in/
ICMR NIRRCH is one of the Regional Resource Centre established by HTAIn Department of Health Research MOHFW since 2017. It is now upgraded to Resource Hub with a mandate to co-ordinate with 3 states Maharashtra, Madhya Pradesh and Goa and 1 Union Territory on matters related to HTA. It received the Best HTA Resource Centre award during the DHR-ICMR Health Research excellence Summit held in November 2024.
It is entrusted with the responsibility to assess cost-effectiveness of new technologies with emphasis on equity, ethical, social and economic issues of a health intervention or health technology, undertake costing exercises on topics suggested by user departments and allocated by HTAIn Secretariat, liase with state and local public health departments to identify topics for conduct of HTA and disseminate results of studies conducted through HTAIn network for adoption into state health programs. The other mandate is to help build capacity and undertake advocacy activities with different stakeholders.
Dr. Beena Joshi, Scientist G, Head Department Operational and Implementation Research is the Principal Investigator. The vision and mandate of NIRRCH as a Resource Hub of HTA is line with that of HTAIn.
Health Technology Assessment (HTA) is defined as the systematic evaluation of properties, effects, and/or impacts of health technology. The word ‘technology’ can include interventions like drugs, devices, diagnostics, treatments, vaccines or health programs. It is a multidisciplinary process that summarizes information about the medical, economic, social, logistic, legal and ethical issues related to the use of health technology in a systematic, transparent, unbiased and robust manner. Its aim is to inform the formulation of safe, effective, health policies that are patient-focused and seek to achieve the best value. The evidence generated through HTA can be used by decision-makers and other stakeholders to support the decision-making process in health care at the policy level thus described as a bridge that connects the world of research to that of policy making.
The results of studies undertaken by the Resource Hub have had very good translational potential and have been meaningfully influenced national health policies and programs especially the National Family Planning program and National Sickle Cell Elimination program to name a few.
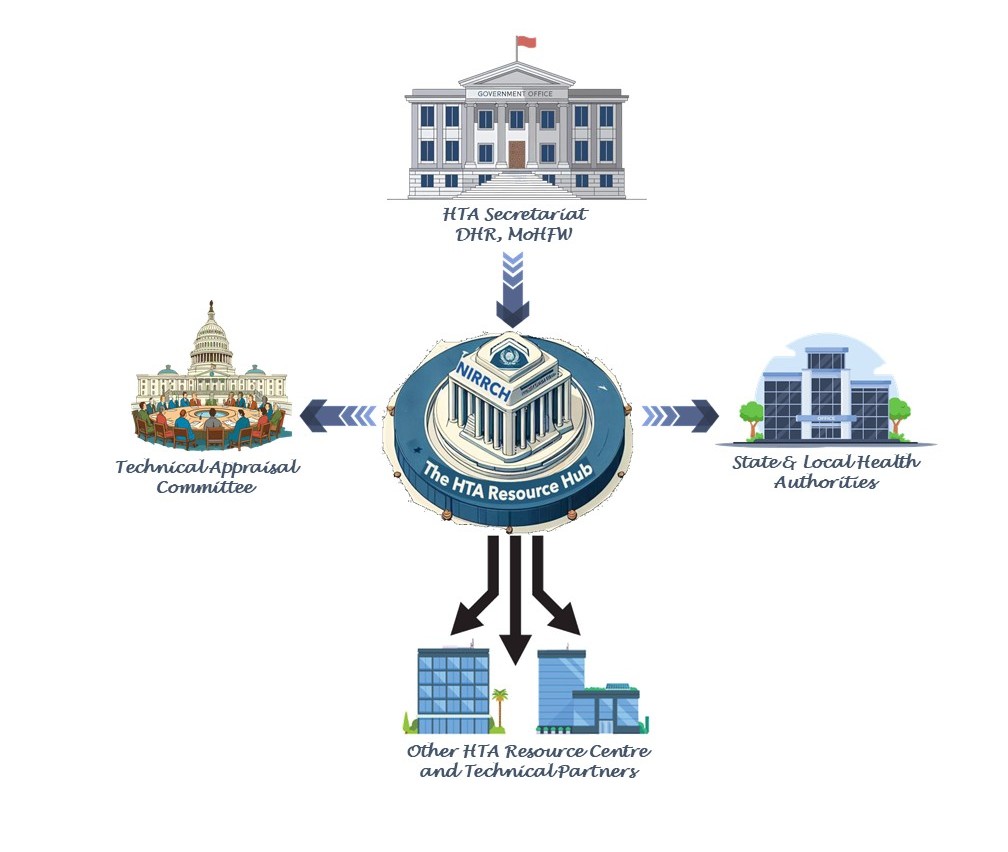
Structure of NIRRCH HTA Regional Resource Hub
Authors :- Joshi Beena, Churasia H, R. R, Shetty S, Kharat N, Begum S
Authors :- Padhan A K, Joshi B, Ambadekar N
Authors :- Srimathi G, Revathy R, Bagepally B, Joshi B
Authors :- Revathy R, Kharat N, Chaurasia H, Shetty S, Begum S, Joshi B
Authors :- Revathy R, Chaurasia H, Shetty S, Joshi B*
Authors :- Beena Joshi, Akshita Vikani.
Link :-https://theindianpractitioner.com/evidence-based-cost-effective-interventions-for-universal-health- coverage/
Authors :- Siddesh Shetty, Kusum Moray, Oshima Sachin, Himanshu Chaurasia, Beena Joshi
Link :- https://www.ijrcog.org/index.php/ijrcog/article/view/12089
Authors :- Siddesh Shetty, Kusum Moray, Himanshu Chaurasia, Beena Joshi
Authors :- Beena Joshi, Siddesh Shetty, Kusum Moray, Oshima Sachin, Himanshu Chaurasia
Link :- https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0256271
Authors :- Kusum Moray, Himanshu Chaurasia, Beena Joshi
Link :- https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0256271
Authors :- Kusum Moray, Himanshu Chaurasia, Oshma Sachin, Beena Joshi
Link :- https://reproductive-health-journal.biomedcentral.com/articles/10.1186/s12978-020-01054-y
Authors :- Kusum Moray, Beena Joshi, Oshima Sachin, Himanshu Chaurasia
Authors :- Beena Joshi, Kusum Moray, Oshima Sachin, Himanshu Chaurasia, Shahina Begum
Link :- https://link.springer.com/article/10.1007/s40258-020-00605-5
Authors :- Beena Joshi, Siddesh Shetty, Kusum Moray, Himanshu Chaurasia, Oshima Sachin
Link :-https://bmcpregnancychildbirth.biomedcentral.com/articles/10.1186/s12884-022-05308-4
Authors :- Namrata Kharat, Revathy Ramachandra, Himansh Chaurasia, Siddesh Shetty, Shahina Begum, Beena Joshi
Link :- https://www.sciencedirect.com/science/article/abs/pii/S2212109923000468
| Sr. No | Title of Project | Funding Agency | From | To |
|---|---|---|---|---|
| 1 | HWC Costing study on RMNCAH+N services/Assessment of RMNCAH+N Service delivery costs, work patterns and efficiency of primary healthcare teams at Ayushman Bharat -Health and wellness centres | UNICEF | 2024 | |
| 2 | Health Technology Assessment To determine Cost-effectiveness of Hydroxyurea oral suspension as prophylaxis for management of Sickle cell Anemia in children | DHR | 2024 | |
| 3 | Cost-effectiveness of point-of-care test for detection of Thalassemia during antenatal screening as an alternative to the HPLC test conducted before twelve weeks of pregnancy. | DHR | 2025 |
| Sr. No | Title of Project | Funding Agency | From | To |
|---|---|---|---|---|
| 1 | Cost-effectiveness of ferric carboxymaltose injection (IFA) to treat iron deficiency anemia among antenatal mothers. | DHR | 2025 | 2025 |
| 2 | Health Technology Assessment of Point of Care Test Kit for Haemophilia and Von Willebrand Disease (vWD) Screening developed at ICMR NIIH (approved by TAC) | HTA | 2024 | 2024 |
| 3 | To estimate the cost of diagnosis of infertility and its management including IVF and quality of life among infertile young couples. | DHR | 2023 | 2024 |
| 4 | To determine Cost-effectiveness of Rapid Diagnostic Tests (Gazalle) in comparison to solubility test followed by HPLC for Sickle Cell Disease/Trait diagnosis among high risk population in India | DHR HTAIn | 2023 | 2023 |
| 5 | To determine Cost-effectiveness of Rapid Diagnostic Tests (Hemo Type Sc, Sickle Scan and HPOS) in comparison to solubility test followed by HPLC for Sickle Cell Disease/Trait diagnosis among high risk population in India | DHR HTAIn | 2022 | 2022 |
| 6 | HTA For strengthening prong 2 intervention of PPTCT program at public health facilities through provision of linked HIV and family planning services | DHR HTAIn | 2021 | 2022 |
| 7 | Health Technology Assessment of intravenous Tranexamic acid use in management of Primary Post-Partum Haemorrhage in India | DHR HTAIn | 2020 | 2020 |
| 8 | Health Technology Assessment (HTA) of Uterine Balloon Tamponades to manage Postpartum Hemorrhage in India | DHR HTAIn | 2020 | 2020 |
| 9 | Health Technology Assessment of Long Acting Reversible Contraceptives in India | DHR HTAIn | 2018 | 2019 |













Scientist ‘G’ & PI
Health Technology Assessment Resouce Hub
ICMR-National Institute for Research in Reproductive and Child Health
Contact No:- +91 224192043 Email Id :- joshib@nirrch.res.in / hta@nirrch.res.in